250,000 litros de alcohol diarios.
Destilería
Producción
Producción Anual
220.000 hectolitros de alcohol.
Productos
Alcohol Hidratado, Alcohol Neutro y Alcohol Anhidro.

GENERALIDADES DE LA FERMENTACIÓN ALCOHÓLICA
El proceso de fermentación alcohólica consiste en la transformación de los azúcares en alcohol. De modo general, el etanol puede ser obtenido por tres formas: vía destilación, vía sintética y vía fermentativa o biológica.
Vía Destilación: se aplica esporádicamente en ciertas regiones vinícolas para el control de precios de determinadas castas de vino de mesa.
Vía Sintética: dependiendo de la disponibilidad se puede obtener de diversas materias primas tales como: eteno, etileno, gases de petróleo, anhídrido carbónico, etc.
Vía Fermentativa o Biológica: se puede producir de materias primas amiláceas, celulósicas o azucaradas como el caldo o melaza de la caña de azúcar. La ecuación de obtención de alcohol de fermentación puede ser resumida por la ecuación de Gay Lussac:
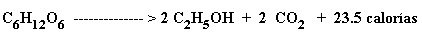
De acuerdo con esta ecuación estequiométrica 100 partes de azúcares producen 51,11 partes de etanol y 48.89 partes de gas carbónico, con desprendimiento de energía.
PREPARACIÓN DEL MOSTO
El mosto debe presentar una concentración de azúcares que sea compatible con la naturaleza de la materia prima, el tipo de la levadura empleada y con el proceso de conducción de la fermentación.
En la práctica, la concentración de mosto es establecida en términos de grados brix, dado que existe una correlación entre el porcentaje de sólidos solubles y el porcentaje de azúcares totales.
La concentración ideal de sólidos en el mosto varia para cada materia prima, siendo generalmente de 14 a 28 °Bx, presentándose las siguientes relaciones con los porcentajes de azúcares:
Mosto de melaza 20 – 28 °Bx 12 – 18 azúcares
Mosto de caldo 14 – 18 °Bx 12 – 18 azúcares
Preparación del Mosto de Melaza
La melaza presenta una concentración de sólidos solubles elevada, necesitándose una dilución a fin de obtener un mosto con concentración ideal de sólidos y consecuentemente de azúcares.
Para preparar un mosto con un brix adecuado, la cantidad de agua en peso que debe ser adicionada a un determinado peso de melaza de concentración conocida, puede ser calculada a través del diagrama conocido “Cruz de Cobenze” o regla de mezclas.
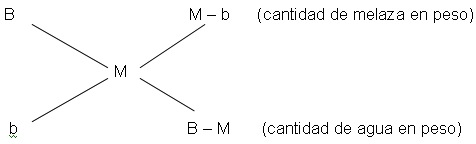
donde:
B: brix de melaza
b: brix del agua
M: brix del mosto
La dilución de la melaza debe ser realizada en tanques denominados “diluidores”, en donde se procura homogenizar lo mejor posible la mezcla agua-melaza. Esta dilución puede ser intermitente a continua.
Corrección de la acidez
Después de la dilución el mosto requiere de una serie de correcciones y una de ellas es la corrección de la acidez, siendo necesario la adición de ácido para bajar el valor de pH entre 4.0 a 5.0. En términos de acidez total, se debe tener un valor entre 2.5 a 3.0 gramos de ácidos sulfúrico por litro de mosto. La acidez favorece el desarrollo de las levaduras y provoca el proceso de inhibición de las bacterias que son agentes contaminantes.
En la práctica se tratan solo los “pies de fermentación”, dado que, cuando se adiciona la semilla al pie de fermentación, el porcentaje de acidez está próximo al ideal..
El uso de ácido sulfúrico no solo da la acidez deseable al mosto, sino que además da una serie de ventajas:
a. descomposición de los nitratos y sulfitos, liberando los radicales volátiles que dificultan el desarrollo del fermento alcohólico;
b. facilitar la inversión de la sacarosa de los mostos que la contienen.
c. es un excelente antiséptico
Corrección de nutrientes
Debido a la inexistencia de nutrientes en materias primas ideales o en algunos casos de desequilibrio entre sus componentes hay necesidad de complementar sales minerales y vitaminas. Estas son el fósforo, nitrógeno, micronutrientes y vitaminas del complejo B.
Para los mostos de melaza es importante la adición de fósforo, dado que la melaza es pobre en este elemento. El fósforo es un elemento trasladador de energía, ligado directamente al ciclo de fermentación y en su ausencia, la fermentación no ocurre. La adición de fosfato diamonico en una dosis de 0.1 gramos por litro de mosto, favorece la actividad de las levaduras y el rendimiento en alcohol.
Otro elemento carente en la melaza es el nitrógeno, siendo su adición necesaria principalmente al inicio de la zafra, cuando se desea favorecer la multiplicación del fermento. El nitrógeno es un elemento de formación plática, importante al crecimiento de la levadura. La adición de sulfato de amonio en una dosis de 0.1 gramos por litros da resultados satisfactorios, dado que beneficia en el proceso de fermentación, rendimiento y calidad del producto final. Debe ser aplicado continuamente durante todo el proceso de fermentación para evitar la formación de aceite de fusel.
Otras sales como el magnesio, manganeso y cobalto en dosis mínimas, son elementos catalizadores de reacciones, siendo por lo tanto favorables al proceso de fermentación.
Las vitaminas son aceleradoras de las acciones enzimáticas de la levadura, influyendo en la velocidad de las fermentaciones.
PROCESOS DE FERMENTACIÓN
Son diversos los procesos de fermentación utilizados para la transformación de los azúcares en alcohol. La variación de cada proceso está unida a la escala de producción, la disponibilidad de los equipos y el dominio de la tecnología. En el caso particular de CATSA, se realiza el siguiente:
Proceso de Propagación de semilla con fermentación batch
Es un procedimiento que tiene excelentes rendimientos, alcanzando parámetros del orden del 92% estequiométrico definido por Gay Lussac, o sea prácticamente el 100% de lo estipulado por Pasteur.
PROPAGACIÓN DE LA SEMILLA EN LA CUBA MADRE
En nuestro proceso el pie del fermentador se desarrolla en la cuba madre, partiendo de una inoculación de alta calidad, la cual ha sido desarrollada en un sistema de propagación de levadura, usándose levaduras del genero Saccharomyces cerevisiae. Esta levadura se inocula en el tanque de propagación y se desarrolla en un medio aséptico, con los nutrientes necesarios, a un P.H entre 4.0-4.5 y con mosto entre 10-12 brix. Una vez que la propagación a concluido, se deben tener los siguientes cuidados:
- Alimentar la cuba madre con mosto de 12 brix hasta que esta se llene en su totalidad.
- Mantener el brix de la cuba madre entre 8.5-9.5, regulando el flujo de mosto.
- Mantenerle suficiente aire a la cuba madre, de tal manera que la población se mantenga en un mínimo de 200 millones de células por mililitro.
- Realizar el primer corte hacia las cubas de fermentación, una vez que la cuba madre esté llena y el brix haya caído a 8.0 (una hora de reposo aproximadamente).
El volumen útil de las cubas de fermentación, se alcanza mediante el llenado, en aproximadamente 12 horas, con mosto entre 14 y 28 brix, dependiendo del contenido de azucares, ya que se debe proyectar un grado alcohólico al final de la fermentación entre 8.0-9.0, para que el proceso de destilación sea eficiente. Los fermentadores son llenados de manera secuencial, ya que la propagación de levadura es continua y se dispone de 20 fermentadores de 250,000 litros de capacidad.
Una vez que se llena el fermentador, se espera a que concluya la fermentación, lo cual se da en unas 12 horas adicionales, para un ciclo total de 24 a 30 horas. El final de la fermentación se determina cuando se consuma la totalidad de los azucares, lo cual se verifica con el laboratorio de control de calidad, aceptándose un máximo de 1.5%.
Cuando la fermentación finaliza, la cuba de fermentación es pasada a la destilación a través de unos tanques de vino, que sirven de compensación o tanques pulmón.
PROCESO DE DESTILACIÓN
En la destilación contínua, el mosto es alimentado continuamente y calentado con vapor de agua a baja presión. El vapor “vapor” arrastra los gases de alcohol, lo que produce una mezcla de gases de alcohol y agua que deben separarse.
Las columnas de destilación constan de varios “platos”, en donde a medida que los vapores ascienden de plato en plato, se va “arrastrando” más alcohol y el agua se va “condensando”, debido a que la temperatura es más baja en la parte superior de la columna que en la base, que es donde se alimenta el vapor.
Una vez separado el producto deseado en forma de vapor, debe recuperarse nuevamente en forma líquida para poder manipularlo. Esto se logra enfriándolo por debajo del punto de ebullición; este fenómeno se llama CONDENSACION y se logra por medio de intercambiadores de calor llamados CONDENSADORES.
Al final de la fermentación el mosto generalmente contiene además del etanol: agua, sales, ácidos grasos, ácidos orgánicos e inorgánicos, acetona, aldehídos, esteres, metanol y aceites de fusel.
En la práctica, a nivel Industrial y para controlar mejor la calidad del producto, se utilizan 2 o más columnas.
Descripción General:
- Capacidad de planta: 240.000 L/día
- Vapor Col. MEG: 150 psig
- Vapor Col.Hidratado: 20 psig.
- Calidad alcohol prod:
- Grado alcohólico: 99.85ºG.L min.
- Humedad: 0.18% max.
- Metanol: 70 ppm max.
- Acidez: 3.0 mg/lt max.
- Fusel: 700.0 p.p.m max.
Funciones Básicas
Columna Purificadora — Extracción de cabezas — Conc. De alcohol — Separación de sólidos
Columna Rectificadora — Extracción de fusel — Separación de sólidos — Conc. Alcohol a 95.
Columna Hidrolizadoraadora — Separa compuestos solubles en agua
Sistema MEG — Deshidratación de alcohol
Columna Purificadora:
En esta columna se separa lo que no es agua y sólidos. El producto obtenido se le conoce con el nombre de “ron”, el cual contiene altos concentraciones de congéneres tales como: aldehídos, alcoholes superiores, esteres. Para condensar los vapores de alcohol, se usa el mosto fermentado o vino que a su vez se calienta antes de entrar a la columna.
Columna Hidroselección:
En esta columna se separa todo compuesto que tiene punto de ebullición más bajo que el alcohol. Para tener una buena separación, es necesario que el alcohol esté diluido a 14-20%.
Se separan los compuestos que son solubles en el agua. El producto superior de esta columna se conoce con el nombre de alcoholes de cabeza.
Columna Rectificación:
En esta columna se purifica el alcohol, eliminando los alcoholes superiores por la parte inferior. Los vapores alcohólicos alcanzan una riqueza de 95-96°GL.
El grado alcohólico depende de la producción y de la relación de reflujo. Si se aumenta la producción baja el grado y viceversa.
En esta columna se extrae el aceite de fusel por la parte baja. Por la parte inferior de la misma se extrae la flemaza, que consiste básicamente en agua caliente.
Columnas de deshidratación:
La destilación extractiva es un proceso químico que consiste en la adición de un determinado compuesto de alto punto de ebullición a una determinada mezcla, de forma que altere la volatilidad relativa de sus componentes, desapareciendo así cualquier mezcla azeotrópica presente en la mezcla original.
Con la ausencia de azeótropos, el solvente puede ser recuperado por una simple destilación, lo que torna la destilación extractiva un sistema de deshidratación mucho mas ventajoso de que los demás procesos azeotrópicos convencionales y mucho mas económico que los procesos existentes.
La diferencia de este proceso consiste en la utilización de los Etilenglicoles como agente extractor para quebrar el azeótropo Etanol/Agua.
Refinaría
Fabricación de Azúcar

Dirección: 125 metros sur de Teletica, Edificio Vista del Parque, 2do piso.
Apartado Postal: 10315-1000 San José
Teléfono: +506 2291-2676
Email: info@catsa.net
Sugerencias o Quejas: info@catsa.net
Dirección: 6.5 kilómetros sureste de la Escuela de Guardia. Liberia, Guanacaste.
Apartado Postal: 56-5000 Liberia.
Teléfono: +506 2690-2500
Email: info@catsa.net
Sugerencias o Quejas: info@catsa.net
Address: 125 mts South from Teletica, Vista del Parque Building, 2nd floor.
Postal Code: 10315-1000 San José
Telephone: +506 2291-2676
Email: info@catsa.net
Comments or Complaints: info@catsa.net
Address: 6.5 kilometer South East from De Guardia School. Liberia, Guanacaste.
Postal Code: 56-5000 Liberia.
Telephone: +506 2690-2500
Email: info@catsa.net
Comments or Complaints: info@catsa.net

Derechos Reservados © 2020 CATSA - Desarrollo Web por CR Webs
